Research
 The pig is due to its anatomy, physiology and genetics a frequently used model in biomedical research. An epitheliochorial type of placentation prevents the prenatal transfer of immunoglobulins and passive immunization of fetuses as it is known in humans. Newborn piglets receive maternal immunoglobulins, immune cells, and cytokines in colostrum to survive in conventional conditions with myriad of microorganisms. They will not survive in these conditions if they are immunoglobulin deprived. In contrast, hysterectomy-derived colostrum-deprived piglets survive in germ-free conditions of gnotobiotic isolators. These germ-free piglets allow us to simulate different states to study host vs. microbiota interactions and interference among different microbial strains. We pay our attention to study 1) preterm gnotobiotic piglets as a model of immunocompromised preterm infants, 2) gastroenterocolitis vs. sepsis caused by Salmonella Typhimurium, and 3) modulation of signaling pathways in sepsis.
The pig is due to its anatomy, physiology and genetics a frequently used model in biomedical research. An epitheliochorial type of placentation prevents the prenatal transfer of immunoglobulins and passive immunization of fetuses as it is known in humans. Newborn piglets receive maternal immunoglobulins, immune cells, and cytokines in colostrum to survive in conventional conditions with myriad of microorganisms. They will not survive in these conditions if they are immunoglobulin deprived. In contrast, hysterectomy-derived colostrum-deprived piglets survive in germ-free conditions of gnotobiotic isolators. These germ-free piglets allow us to simulate different states to study host vs. microbiota interactions and interference among different microbial strains. We pay our attention to study 1) preterm gnotobiotic piglets as a model of immunocompromised preterm infants, 2) gastroenterocolitis vs. sepsis caused by Salmonella Typhimurium, and 3) modulation of signaling pathways in sepsis.
Preterm gnotobiotic piglets as a model of immunocompromised preterm infants
 They are more endangered by infections than their term counterparts. We colonize the gastrointestinal tract with probiotics and their candidates to verify their safety for the underdeveloped organism. The intestinal histology, transcriptions of tight junction proteins claudin-1, claudin-2, and occludin, and local and systemic levels of inflammatory cytokines (mainly IL-8, TNF-α, IL-10, and HMGB1) serves to evaluate possible pathological changes (Splichalova et al, 2019Splichalova A, Jenistova V, Splichalova Z, Splichal I
They are more endangered by infections than their term counterparts. We colonize the gastrointestinal tract with probiotics and their candidates to verify their safety for the underdeveloped organism. The intestinal histology, transcriptions of tight junction proteins claudin-1, claudin-2, and occludin, and local and systemic levels of inflammatory cytokines (mainly IL-8, TNF-α, IL-10, and HMGB1) serves to evaluate possible pathological changes (Splichalova et al, 2019Splichalova A, Jenistova V, Splichalova Z, Splichal I
Colonization of preterm gnotobiotic piglets with probiotic Lactobacillus rhamnosus GG and its interference with Salmonella Typhimurium.
Clin Exp Immunol 2018, 195:381-394.;Splichalova et al. 2018Splichalova A, Slavikova V, Splichalova Z, Splichal I
Preterm life in sterile conditions: A study on preterm, germ-free piglets.
Front Immunol 2019, 9:220.).
Gastroenterocolitis vs. sepsis caused by Salmonella Typhimurium
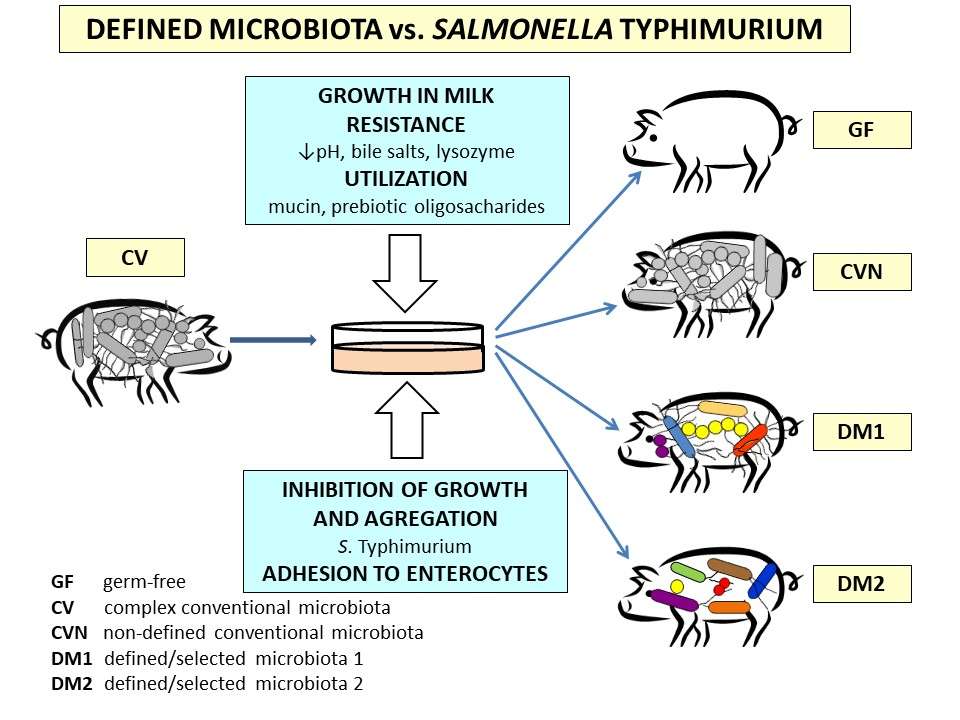 S. Typhimurium is an enteric pathogen that causes in the human and pig salmonellosis (gastroenterocolitis). However, in immunocompromised individuals, it can provoke life endangering systemic illness. Suitable bacterial interferences, e.g., E. coli Nissle 1917 can prevent systemic spread of Salmonella and sepsis. Different Δrfa isogenic S. Typhimurium strain LT2 mutants are used to study bacterial interferences to evaluate the importance of lipopolysaccharide completeness on bacterial virulence too. The intestinal histology, tight junction proteins, and inflammatory cytokines describe pathological changes caused by Salmonella infection.
S. Typhimurium is an enteric pathogen that causes in the human and pig salmonellosis (gastroenterocolitis). However, in immunocompromised individuals, it can provoke life endangering systemic illness. Suitable bacterial interferences, e.g., E. coli Nissle 1917 can prevent systemic spread of Salmonella and sepsis. Different Δrfa isogenic S. Typhimurium strain LT2 mutants are used to study bacterial interferences to evaluate the importance of lipopolysaccharide completeness on bacterial virulence too. The intestinal histology, tight junction proteins, and inflammatory cytokines describe pathological changes caused by Salmonella infection.
Modulation of signaling pathways in sepsis
Toll-like receptors (TLRs) 2, 4, 5, and 9 and Nod-like receptors (NLRs) NOD1 and NOD2 sense pathogen-associated molecular patterns. Transcriptions of adaptor molecules MyD88 and TRIF and different proteins of nuclear factor κB family with related molecules are other targets of our interest. Possible modifications in their transcriptions by probiotics or biologically active molecules to prevent the deleterious effect of “cytokine storm” could be ways of ameliorating of sepsis consequences.