Research
Development of B lymphocytes
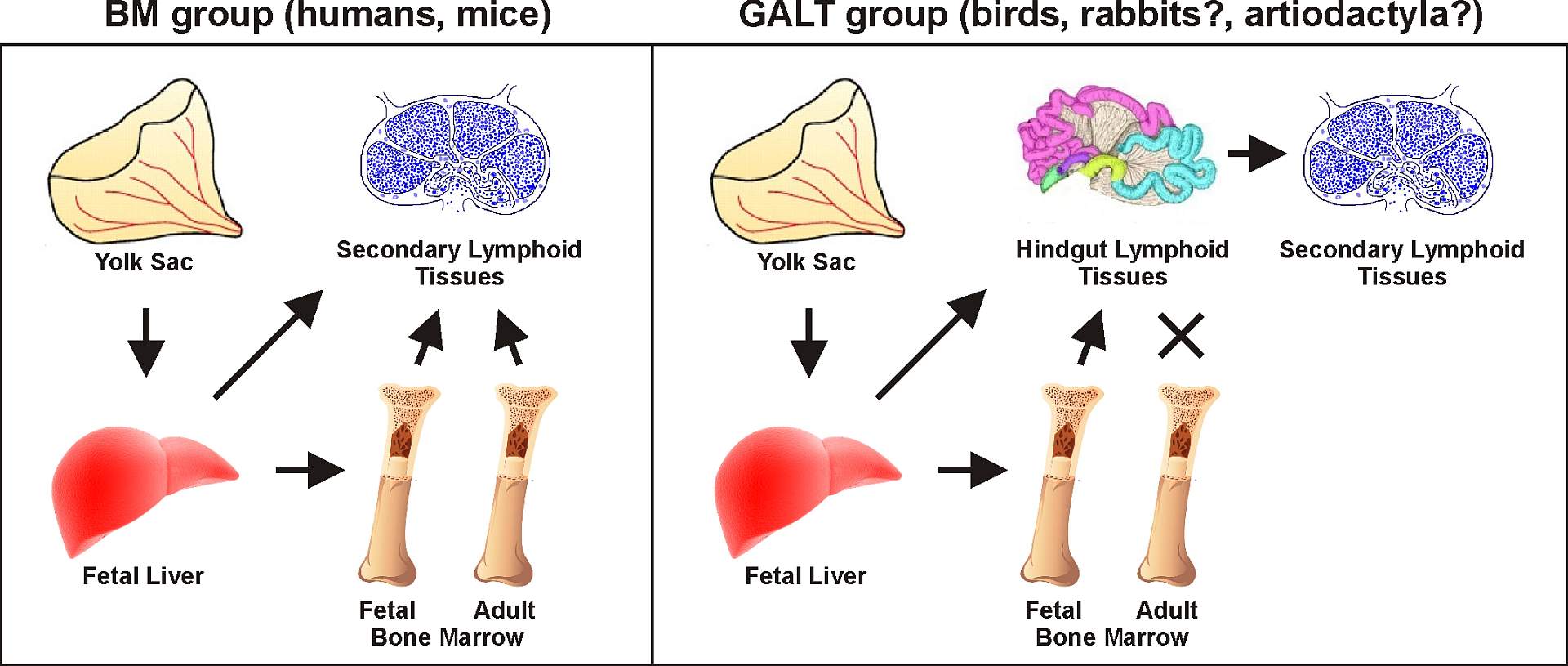 Based on studies of sheep, the ileal Peyer’s patches (IPP) have been regarded as a type of primary lymphoid tissue similar to the bursa of Fabricius in chicken. It was assumed that there is massive antigen-independent B cell repertoire development in the IPP and that diversification occurs by somatic hypermutation and/or gene conversion. This concept has permeated other scientific reports, reviews and immunology textbooks for more than 20 years and due to the similarity in the organization of IPP, was broadened also to other species that include members of artiodactyls (including pig) and probably some cetaceans and carnivores. However, our previous data were in conflict with this presumption (Sinkora and Butler, 2009Sinkora M and Butler JE
Based on studies of sheep, the ileal Peyer’s patches (IPP) have been regarded as a type of primary lymphoid tissue similar to the bursa of Fabricius in chicken. It was assumed that there is massive antigen-independent B cell repertoire development in the IPP and that diversification occurs by somatic hypermutation and/or gene conversion. This concept has permeated other scientific reports, reviews and immunology textbooks for more than 20 years and due to the similarity in the organization of IPP, was broadened also to other species that include members of artiodactyls (including pig) and probably some cetaceans and carnivores. However, our previous data were in conflict with this presumption (Sinkora and Butler, 2009Sinkora M and Butler JE
The ontogeny of the porcine immune system..
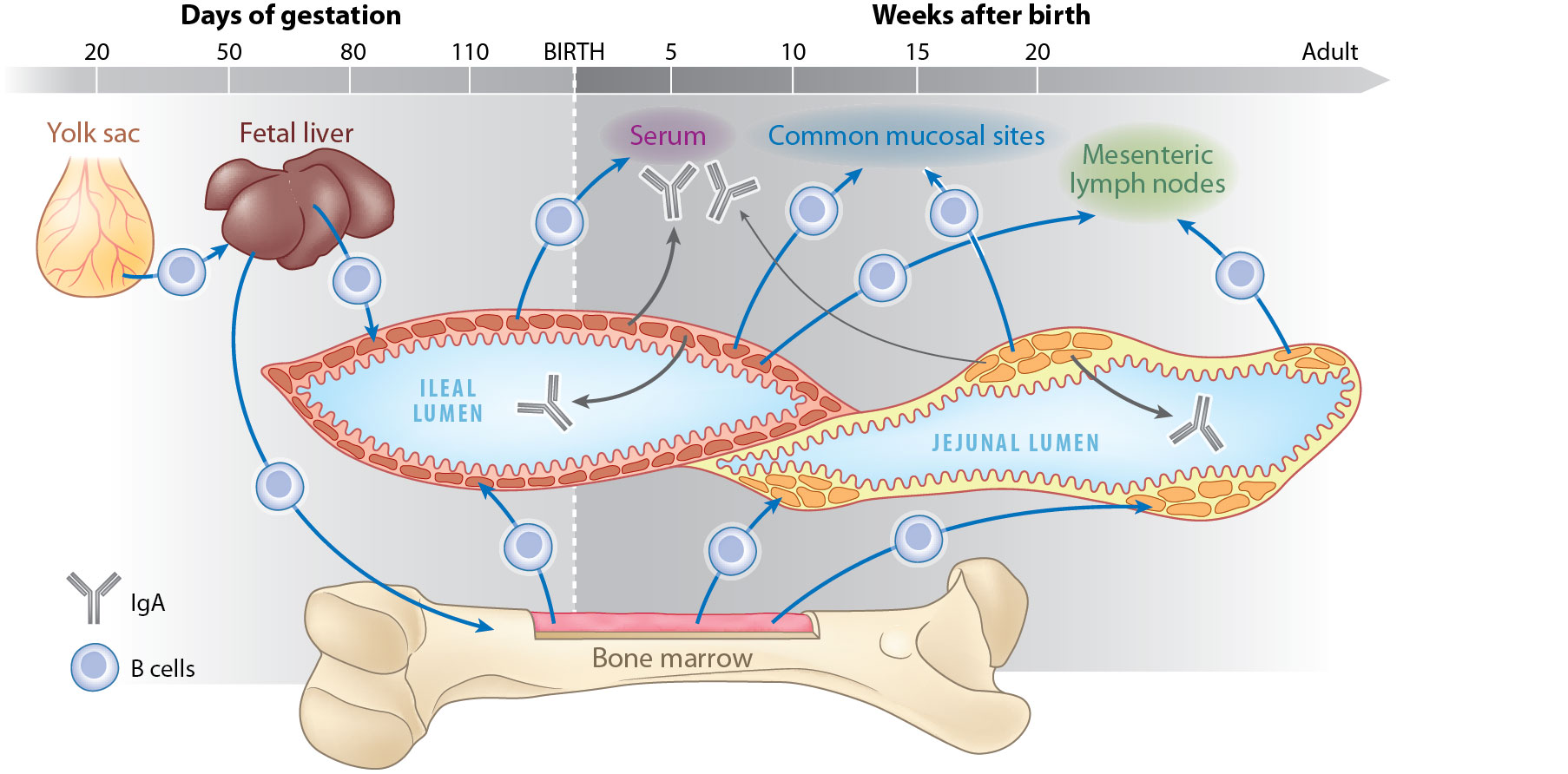
Dev Comp Immunol. 2009, 33: 273-283.). For this reason we have designed a series of experiments using germ-free and gnotobiotic piglets to demonstrate dispensability of ileal Peyer's patches for B cell development. The results disprove the concept that porcine ileal Peyer's patches are a significant source of B cells, are required for systemic B cell generation and maintenance and are a site of B cell lymphogenesis in swine (Butler and Sinkora, 2013Butler JE and Sinkora M
The enigma of the lower gut-associated lymphoid tissue (GALT)..
J Leukoc Biol. 2013, 94: 259-270.). We have also clearly shown that maturation of B cells in ileal Peyer's patches is antigen-dependent and it is heavily dependent on colonization (Potockova et al., 2015Potockova H, Sinkorova J, Karova K, Sinkora M
The distribution of lymphoid cells in the small intestine of germ-free and conventional piglets..
Dev Comp Immunol. 2015, 51: 99-107.; Butler et al., 2017Butler JE, Wertz N, Sinkora M
Antibody Repertoire Development in Swine..
Annu Rev Anim Biosci. 2017, 5: 255-279.), which indicates that ileal Peyer's patches are a secondary lymphoid tissue that appear important in immune responses to colonizing bacteria. On the other hand, using flow cytometry sorting and semi-quantitative PCR detection of rearrangement-specific transcripts and DNA products we show that the bone marrow is a major B lymphopoietic organ of pigs (Sinkora and Sinkorova, 2014Sinkora M and Sinkorova J
B cell lymphogenesis in swine is located in the bone marrow..
J Immunol. 2014, 193: 5023-5032.). Further findings allow us to clarify the progression and differentiation pathways of B cell maturation in the bone marrow and showed that the immunoglobulin light chain precedes the heavy chain gene rearrangement during porcine B cells development (Sinkora et al., 2017Sinkora M, Sinkorova J, Stepanova K
Ig Light Chain Precedes Heavy Chain Gene Rearrangement during Development of B Cells in Swine..
J Immunol. 2017, 198: 1543-1552.). Our most recent work concerns the order of immunoglobulin light chain κ and λ usage in primary and secondary lymphoid tissues in germ-free and conventional piglets (Štěpánová et al., 2022Katerina Stepanova, Jana Sinkorova, Dagmar Srutkova, Marek Sinkora Jr., Simon Sinkora, Igor Splichal, Alla Splichalova, John E. Butler, Marek Sinkora
The order of immunoglobulin light chain κ and λ usage in primary and secondary lymphoid tissues of germ-free and conventional piglets.). A number of these experiments have demonstrated that pigs use an alternative B cell development pathway compared to mice and humans and that the majority of λ+ B cells are generated earlier in pigs (Šinkora et al., 2020Marek Sinkora, Katerina Stepanova, Jana Sinkorova
Immunoglobulin light chain κ precedes λ rearrangement in swine but a majority of λ+ B cells are generated earlier.). The mechanism is based on a preferential rearrangement of authentic light chains so that species such as the pig become independent of surrogate light chains (Šinkora et al., 2022aMarek Sinkora, Katerina Stepanova, Jana Sinkorova
Consequences of the different order of immunoglobulin gene rearrangements in swine.). According to these results, there are at least two groups of mammals: one uses a pre-BCR-driven developmental pathway for B cell generation (such as mice and humans) and the second group uses a pre-BCR-independent pathway (such as pigs). These findings are important for understanding the evolutionary approaches, redundancy and efficiency of B-cell generation, and dependencies on other regulatory factors (Šinkora et al., 2022bMarek Sinkora, Katerina Stepanova, John E. Butler, Marek Sinkora Jr, Simon Sinkora, Jana Sinkorova
Comparative Aspects of Immunoglobulin Gene Rearrangement Arrays in Different Species.
Front. Immunol., 11 February 2022.).
Peripheral B lymphocytes
 Our studies of B lymphocytes in pigs also involve characterization of B cell maturation in the periphery. Ontogenetic and in vitro culture studies, analysis of cell size, expression of CD11b and class-switched phenotype together with proliferation and cell death measurement, revealed four subpopulations of functionally different B cells in the periphery according to expression of CD2 and CD21 (Sinkora et al., 2013Sinkora M, Stepanova K, Sinkorova J
Our studies of B lymphocytes in pigs also involve characterization of B cell maturation in the periphery. Ontogenetic and in vitro culture studies, analysis of cell size, expression of CD11b and class-switched phenotype together with proliferation and cell death measurement, revealed four subpopulations of functionally different B cells in the periphery according to expression of CD2 and CD21 (Sinkora et al., 2013Sinkora M, Stepanova K, Sinkorova J
Different anti-CD21 antibodies can be used to discriminate developmentally and functionally different subsets of B lymphocytes in circulation of pigs..
Dev Comp Immunol. 2013, 39: 409-418.). Moreover, we have shown that CD21 molecules are always present on all mature B cells but can be expressed in at least two different forms CD21a and CD21b. We have also described that end-stage B cells can express these different form of CD21, which can be significant for their function, and that expression of these forms can be used for monitoring of B cell maturation during infection (Sinkora et al., 2014Sinkora M, Butler JE, Lager KM, Potockova H, Sinkorova J
The comparative profile of lymphoid cells and the T and B cell spectratype of germ-free piglets infected with viruses SIV, PRRSV or PCV2..
Vet Res. 2014, 45:91., Butler et al., 2014Butler JE, Lager KM, Golde W, Faaberg KS, Sinkora M, Loving C, Zhang YI
Porcine reproductive and respiratory syndrome (PRRS): an immune dysregulatory pandemic..
Immunol Res. 2014, 59: 81-108.) and colonization (Potockova et al., 2015Potockova H, Sinkorova J, Karova K, Sinkora M
The distribution of lymphoid cells in the small intestine of germ-free and conventional piglets..
Dev Comp Immunol. 2015, 51: 99-107.).
Development of αβ T lymphocytes
 In comparison to other species, swine express some differences in the αβ T cell pool that may reflect modifications in T lymphocyte ontogeny and differentiation (Sinkora et al., 2005aSinkora M, Butler JE, Holtmeier W, Sinkorova J
In comparison to other species, swine express some differences in the αβ T cell pool that may reflect modifications in T lymphocyte ontogeny and differentiation (Sinkora et al., 2005aSinkora M, Butler JE, Holtmeier W, Sinkorova J
Lymphocyte development in fetal piglets: facts and surprises..
Vet Immunol Immunopathol. 2005, 108(1-2): 177-184.) . For this reason, we have characterized development of αβ T cell in thymus, which is the primary lymphocyte tissue for these cells. Despite unique features of peripheral αβ T cells, developing thymocytes in pigs mostly resemble those in other species (Sinkora et al., 2000Sinkora M, Sinkora J, Reháková Z, Butler JE
Early ontogeny of thymocytes in pigs: sequential colonization of the thymus by T cell progenitors..
J Immunol. 2000, 165: 1832-1839) . However, long gestation in swine allows us to describe successive colonization waves of thymus by hemopoietic progenitors during embryogenesis. We have also shown that the influx of thymocyte progenitors is discontinuous. This is not possible in mice because waves of thymic colonization overlap. The data suggest that porcine αβ thymocytes require about 15 days to fully differentiate, while γδ thymocytes do so in less than 3 days. We also observed that the porcine IL-2Rα (CD25), a typical differentiation marker of pre-T cells in mice and humans, was not expressed on thymocyte precursors in pigs and could only be found on mature thymocytes. Finally, surface immunophenotyping and cell cycle analysis of thymocyte subsets allowed us to propose a model for αβ T cell lymphopoiesis in swine.
Development of γδ T lymphocytes
 Based on the expression of cell surface markers we have characterized the ontogeny of γδ thymocytes. Using the precise definition of developmental pathways for conventional γδ thymocytes (Sinkora et al., 2005bSinkora M, Sinkorová J, Holtmeier W
Based on the expression of cell surface markers we have characterized the ontogeny of γδ thymocytes. Using the precise definition of developmental pathways for conventional γδ thymocytes (Sinkora et al., 2005bSinkora M, Sinkorová J, Holtmeier W
Development of γδ thymocyte subsets during prenatal and postnatal ontogeny..
Immunology. 2005, 115: 544-555.) , the populations of γδ thymocytes was found to have no counterpart in the periphery (Sinkora et al., 2007Sinkora M, Sinkorová J, Cimburek Z, Holtmeier W
Two groups of porcine TCRγδ+ thymocytes behave and diverge differently..
J Immunol. 2007, 178: 711-719.) . In total, 12 subpopulations of porcine γδ thymocytes based on their expression of CD1, CD2, CD4, CD8 and CD45RC were characterized. Our observation also indicates that two subsets of peripheral γδ T lymphocytes differing in MHC-II expression exist, depend of their origin during ontogeny. We have proposed that one subset acquires CD8αα in the thymus while the second acquires CD8αα as a result of stimulation in the periphery.
Peripheral γδ T lymphocytes in swine
 In order to characterize peripheral γδ T cells in swine we investigated expressions of CD25, CD11b, CD52, SWC7, MHC-II and the family of CD45 molecules on these cells. We have found that they differ depending on bacterial colonization, age of animals and/or status of cell activation (Stepanova and Sinkora, 2012Stepanova K and Sinkora M
In order to characterize peripheral γδ T cells in swine we investigated expressions of CD25, CD11b, CD52, SWC7, MHC-II and the family of CD45 molecules on these cells. We have found that they differ depending on bacterial colonization, age of animals and/or status of cell activation (Stepanova and Sinkora, 2012Stepanova K and Sinkora M
The expression of CD25, CD11b, SWC1, SWC7, MHC-II, and family of CD45 molecules can be used to characterize different stages of γδ T lymphocytes in pigs..
Dev Comp Immunol. 2012, 36: 728-740.) . These studies imply that CD2/CD8 subsets of porcine γδ T cells may represent functionally different cells. For this reason, these subpopulations of γδ T lymphocytes were studied in more detail. Sorting and following in vitro cultivation showed that CD2 expression can be used for definition of two lineages of γδ cells (Stepanova and Sinkora, 2013Stepanova K and Sinkora M
Porcine γδ T lymphocytes can be categorized into two functionally and developmentally distinct subsets according to expression of CD2 and level of TCR..
J Immunol. 2013, 190: 2111-2120.; Potockova et al., 2015Potockova H, Sinkorova J, Karova K, Sinkora M
The distribution of lymphoid cells in the small intestine of germ-free and conventional piglets..
Dev Comp Immunol. 2015, 51: 99-107.) . Because CD2— γδ T cells are missing in the blood of humans and mice, but are obvious in other members of γδ high species such as ruminants and birds, our findings support the idea that circulating CD2— γδ T cells are a specific lineage (Sinkora and Butler, 2016Sinkora M and Butler JE
Progress in the use of swine in developmental immunology of B and T lymphocytes..
Dev Comp Immunol. 2016, 58: 1-17.).
In addition to the work concerning porcine γδ T cells we have also investigated murine γδ T cells expressing canonical Vδ1-Dδ2-Jδ2 chain, which completely lacks junctional diversity (Holtmeier et al., 2010Holtmeier W, Gille J, Zeuzem S, Sinkora M
Distribution and development of the postnatal murine Vδ1 T-cell receptor repertoire..
Immunology. 2010, 131: 192-201.). We showed that γδ T cells expressing this canonical TCRδ chain are not unique to the skin and reproductive sites as expected from other mouse studies. We also discovered other γδ T cells expressing fetal type Vδ1 chains, which were shared among different organs and animals. These findings indicate that γδ T cells expressing conserved Vδ1 chains continuously recirculate throughout the organism and rapidly respond to stress-induced self antigens.
Involvement of porcine lymphocytes in viral diseases
 Our laboratory also participated in studies of the porcine immune system during experimental infection by swine influenza virus (SIV), porcine reproductive and respiratory syndrome virus (PRRSV) and porcine circovirus type 2 (PCV2). Germ-free piglets were used since their response could be directly correlated to the viral infection. Using the phenotypic profile of T, B and NK cells and their subsets and utilizing our knowledge about these lymphoid cells in swine we have shown that PRRSV and PCV2 negatively modulate the host immune system by different mechanisms which may explain their persistence (Sinkora et al., 2014Sinkora M, Butler JE, Lager KM, Potockova H, Sinkorova J
Our laboratory also participated in studies of the porcine immune system during experimental infection by swine influenza virus (SIV), porcine reproductive and respiratory syndrome virus (PRRSV) and porcine circovirus type 2 (PCV2). Germ-free piglets were used since their response could be directly correlated to the viral infection. Using the phenotypic profile of T, B and NK cells and their subsets and utilizing our knowledge about these lymphoid cells in swine we have shown that PRRSV and PCV2 negatively modulate the host immune system by different mechanisms which may explain their persistence (Sinkora et al., 2014Sinkora M, Butler JE, Lager KM, Potockova H, Sinkorova J
The comparative profile of lymphoid cells and the T and B cell spectratype of germ-free piglets infected with viruses SIV, PRRSV or PCV2..
Vet Res. 2014, 45:91.; Butler et al., 2014Butler JE, Lager KM, Golde W, Faaberg KS, Sinkora M, Loving C, Zhang YI
Porcine reproductive and respiratory syndrome (PRRS): an immune dysregulatory pandemic..
Immunol Res. 2014, 59: 81-108.). The most expressive differences were observed as regards the B cells and their subsets: while massive overproduction of immunoglobulins was observed in the PRRSV infection (up to 20mg/ml), the PCV2 infection was characteristic of their decrease. Molecular analysis showed an expansion of dominant B cell clones in PRRSV infection as a result of T-independent proliferation of naive B cells without any repertoire diversification and affinity maturation. The PCV2 infection was characteristic of αβ T cell dysregulation expressed by increased numbers of cytotoxic T cells at the expenses of helper T cells. Our results explain overall lymphoid hyperplasia, hypergammaglobulinemia and autoimmunity in the case of PRRSV infection and also immunosuppressive effect of PCV2 infection. New findings in a field of PRRSV lead us to recent hypothesis that immune dysregulation is caused by altered thymocyte development when PRRSV-specific T cells are selectively eliminated, which cause their absence in the periphery (Butler et al., 2019Butler JE, Sinkora M, Wang G, Stepanova K, Li Y, Cai X
Perturbation of thymocyte development underlies the PRRS pandemic: A testable hypothesis. Front Immunol. 2019, in press.). The "hole" in T cell repertoire allows acceptation of PRRSV epitopes as self-antigens so that specific immune response against PRRSV is missing. Without adequate PRRSV-specific T cells, the selection of B cells capable of producing high affinity neutralizing antibodies is impaired, as is the generation of cytotoxic T cells (Šinkora et al., 2023Marek Sinkora, Miroslav Toman, Katerina Stepanova, Hana Stepanova, Lenka Leva, Jana Sinkorova, Romana Moutelikova, Jiri Salat, Dagmar Srutkova, Martin Schwarzer, Simon Sinkora, Helena Kupcova Skalnikova, Katerina Nechvatalova, Tomas Hudcovic, Petra Hermanova, Sarka Pfeiferova, Mirka Kratochvilova, Lenka Kavanova, Blanka Dusankova, Marek Jr. Sinkora
The mechanism of immune dysregulation caused by porcine reproductive and respiratory syndrome virus (PRRSV).
Front. Immunol., 11 February 2022.). The results show how a respiratory virus, which primarily infects and destroys myelomonocytic cells, has developed strategies to disrupt the immune system. Since modified live vaccines against PRRSV cause the same immune system dysregulation, they should be considered potentially dangerous (Štěpánová et al., 2024Katerina Stepanova, Miroslav Toman, Jana Sinkorova, Simon Sinkora, Sarka Pfeiferova, Helena Kupcova Skalnikova, Salim Abuhajiar, Romana Moutelikova, Jiri Salat, Hana Stepanova, Katerina Nechvatalova, Lenka Leva, Petra Hermanova, Mirka Kratochvilova, Blanka Dusankova, Marek Sinkora Jr, Vratislav Horak, Tomas Hudcovic, John E. Butler, Marek Sinkora
Modified live vaccine strains of porcine reproductive and respiratory syndrome virus cause immune system dysregulation similar to wild strains.
Front. Immunol., 12 January 2024; Toman et al., 2024Miroslav T., Nechvátalová K., Štěpánová K., Šinkora M.
Dysregulace imunitního systému selat po infekci virem PRRS – nový pohled na možnosti profylaxe této infekce.
Veterinářství 2024;74(2):98-101 ).
Involvement of porcine lymphocytes in tumors
 Using a porcine model, we also describe Melanoma-Associated T-lymphocytes (MATL) in peripheral blood that increase during melanoma regression. These MATL possess the CD4+CD8hi phenotype and they have their direct counterparts in Tumor Infiltrating Lymphocytes (TIL) isolated from melanoma loci. Both MATL and TIL have a similar expression of selected markers indicating that they represent effector/memory αβ T-cell subset. Importantly, TIL isolated from different pigs and different melanoma loci among the same pig have similar phenotype and distribution, indicating that the composition of the MATL and TIL compartment is identical. Analysis of sorted cells from regressing pigs revealed a unique MATL subpopulation with mono-specific T-cell receptor that was further analyzed by sequencing. These results indicate that pigs regressing melanomas possess a characteristic population of recirculating T-cells playing a role in tumor control and regression (Cizkova et al., 2019Cizkova J, Sinkorova Z, Strnadova K, Cervinkova M, Horak V, Sinkora J, Stepanova K, Sinkora M
Using a porcine model, we also describe Melanoma-Associated T-lymphocytes (MATL) in peripheral blood that increase during melanoma regression. These MATL possess the CD4+CD8hi phenotype and they have their direct counterparts in Tumor Infiltrating Lymphocytes (TIL) isolated from melanoma loci. Both MATL and TIL have a similar expression of selected markers indicating that they represent effector/memory αβ T-cell subset. Importantly, TIL isolated from different pigs and different melanoma loci among the same pig have similar phenotype and distribution, indicating that the composition of the MATL and TIL compartment is identical. Analysis of sorted cells from regressing pigs revealed a unique MATL subpopulation with mono-specific T-cell receptor that was further analyzed by sequencing. These results indicate that pigs regressing melanomas possess a characteristic population of recirculating T-cells playing a role in tumor control and regression (Cizkova et al., 2019Cizkova J, Sinkorova Z, Strnadova K, Cervinkova M, Horak V, Sinkora J, Stepanova K, Sinkora M
The role of αβ T-cells in spontaneous regression of melanoma tumors in swine..
Dev Comp Immunol. 2019, 92: 60-68.).
Involvement of porcine lymphocytes in cystic fibrosis
 Our laboratory also participated in the investigation of inflammatory cells that participate in the destruction of the pancreas during the course of cystic fibrosis (CF). In CF, pancreatic disease begins in utero and progresses over time to the complete destruction of the beta cells. This is an unusual type of autoimmune inflammation that develops in fetal life. The study was performed on the Cystic Fibrosis Transmembrane conductance Regulator (CFTR) gene knock-out pig model. Unlike mice, these CFTR–/– pigs develop pathological changes similar to humans. We discovered the activation of both the innate and adaptive immune systems during destruction of pancreas indicating that immune reaction may be initiated during fetal life, in a period of time when only tolerance should be expected (Abu-El-Haija et al., 2011Abu-El-Haija M, Sinkora M, Meyerholz DK, Welsh MJ, McCray PB Jr, Butler J, Uc A
Our laboratory also participated in the investigation of inflammatory cells that participate in the destruction of the pancreas during the course of cystic fibrosis (CF). In CF, pancreatic disease begins in utero and progresses over time to the complete destruction of the beta cells. This is an unusual type of autoimmune inflammation that develops in fetal life. The study was performed on the Cystic Fibrosis Transmembrane conductance Regulator (CFTR) gene knock-out pig model. Unlike mice, these CFTR–/– pigs develop pathological changes similar to humans. We discovered the activation of both the innate and adaptive immune systems during destruction of pancreas indicating that immune reaction may be initiated during fetal life, in a period of time when only tolerance should be expected (Abu-El-Haija et al., 2011Abu-El-Haija M, Sinkora M, Meyerholz DK, Welsh MJ, McCray PB Jr, Butler J, Uc A
An activated immune and inflammatory response targets the pancreas of newborn pigs with cystic fibrosis..
Pancreatology. 2011, 11: 506-515.).